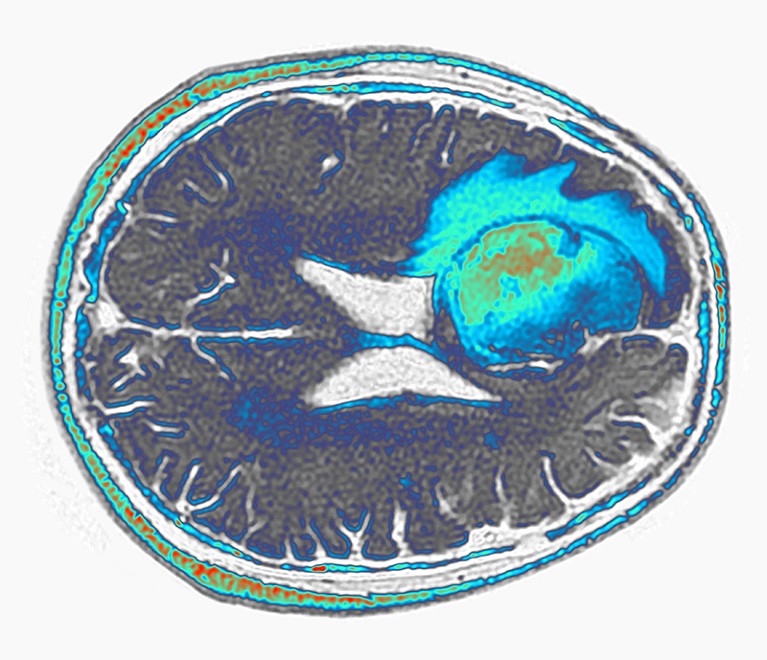
A glioblastoma (green and blue, artificially coloured) grows in the frontal lobe of a person’s brain.Credit: Pr Michel Brauner, ISM/Science Photo Library
Two preliminary studies suggest that next-generation engineered immune cells show promise against one of the most feared forms of cancer.
A pair of papers published on 13 March, one in Nature Medicine1 and the other in The New England Journal of Medicine2, describe the design and deployment of immune cells called chimeric antigen receptor T (CAR T) cells against glioblastoma, an aggressive and difficult-to-treat form of brain cancer. The average length of survival for people with this tumour is eight months.
Both teams found early hints of progress using CAR T cells that target two proteins made by glioblastoma cells, thereby marking those cells for destruction. CAR T cells are currently approved for treating only blood cancers, such as leukaemia, and are typically engineered to home in on only one target. But these results add to mounting evidence that CAR T cells could be modified to treat a wider range of cancers.
“It lends credence to the potential power of CAR T cells to make a difference in solid tumours, especially the brain,” says Bryan Choi, a neurosurgeon at Massachusetts General Hospital in Boston, and a lead author of the New England Journal of Medicine study. “It adds to the excitement that we might be able to move the needle.”
A highly lethal tumour
Glioblastomas offer a formidable challenge. Fast-growing glioblastomas can mix with healthy brain cells, forming diffuse tumours that are difficult to remove surgically. Surgery, chemotherapy and radiation therapy are typically the only treatment options for these tumours, and tend to produce short-lived, partial responses.
Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
In CAR-T therapy, a person’s own T cells are removed from their body and kitted out with proteins that help the cells home in on tumours. The souped-up cells are then reinfused into the body.
In the past few years, researchers have been developing CAR T cells that target specific molecules made by some glioblastomas. The two papers take this a step farther by designing CAR T cells that target not one type of molecule but two.
In one approach, Choi and his colleagues designed CAR T cells to latch onto a mutated form of a protein called epidermal growth factor receptor (EGFR), which is produced by some glioblastoma cells. The CAR T cells also secreted antibodies that bind to both T cells and to the unmutated form of EGFR, which is not typically produced by brain cells but, often by glioblastoma cells. The result is a CAR-T therapy that unleashes the immune system against cells that express either the mutated or the unmutated form of EGFR.
Choi and his team administered these cells to three adults with glioblastoma. Tumours appeared to shrink in all three, but later recurred. One man who received the treatment, however, had a response that lasted for more than six months.
Seven months and counting
The other team, led by Stephen Bagley, a neuro-oncologist at the University of Pennsylvania Perelman School of Medicine in Philadelphia, used CAR T cells that target both EGFR and another protein found in glioblastomas called interleukin-13 receptor alpha 2. Tumours appeared to shrink in all six of the people that were treated. One participant’s glioblastoma began to grow again within a month, but another has not shown signs of tumour progression for seven months, says Bagley. Of the remaining four participants, one left the trial, and tumours have not rebounded in the remaining three, but they are within six months of treatment.
The results are promising, but the goal is to generate longer-lasting responses, says Bagley. It was exciting, he says, to watch tumours shrink in the first day after CAR-T therapy. “We hadn’t seen that before,” he says. “We were thrilled.”
But the excitement faded as participants relapsed after treatment. “It’s very humbling to go on that rollercoaster ride,” he says. “One week you feel like you’ve made a real difference in their lives, and the next week the tumour is back again.”
Versatile T cells
The field will eagerly await more results, says Sneha Ramakrishna, a paediatric oncologist at Stanford Medicine in California. The size of glioblastomas is notoriously difficult to measure because of their diffuse shape, and apparent changes in tumour size could be affected by inflammation following surgery to administer the CAR T cells directly into the brain.
But the images are impressive, and measures of tumour RNA in Choi and colleagues’ study suggest that the tumours might have indeed shrunk, says Ramakrishna. And constructing CAR T cells with multiple targets could ultimately yield long-lasting therapies, she says, by making it more difficult for cancer cells to develop ways to resist the therapy.
“I’m looking forward to seeing what they do over time,” she says. “I hope that as we get more experience, we can learn how to make the right CAR for our patients.”